| Clinical application | Clinical auxiliary diagnosis of endometrial carcinoma |
| Detection gene | PCDHGB7 |
| Sample type | Female cervical specimens |
| Test method | Fluorescence quantitative PCR technology |
| Applicable models | ABI7500 |
| Packing specification | 48 tests/kit |
| Storage Conditions | Kit A should be stored at 2-30℃ Kit B should be stored at -20±5℃ Valid for up to 12 months. |


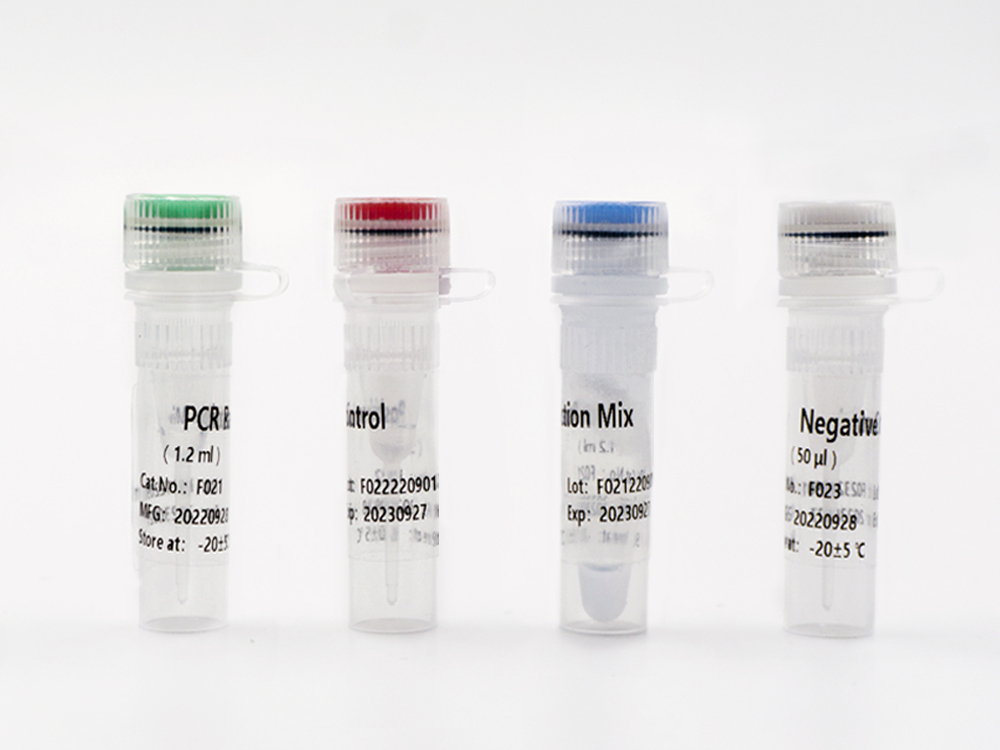
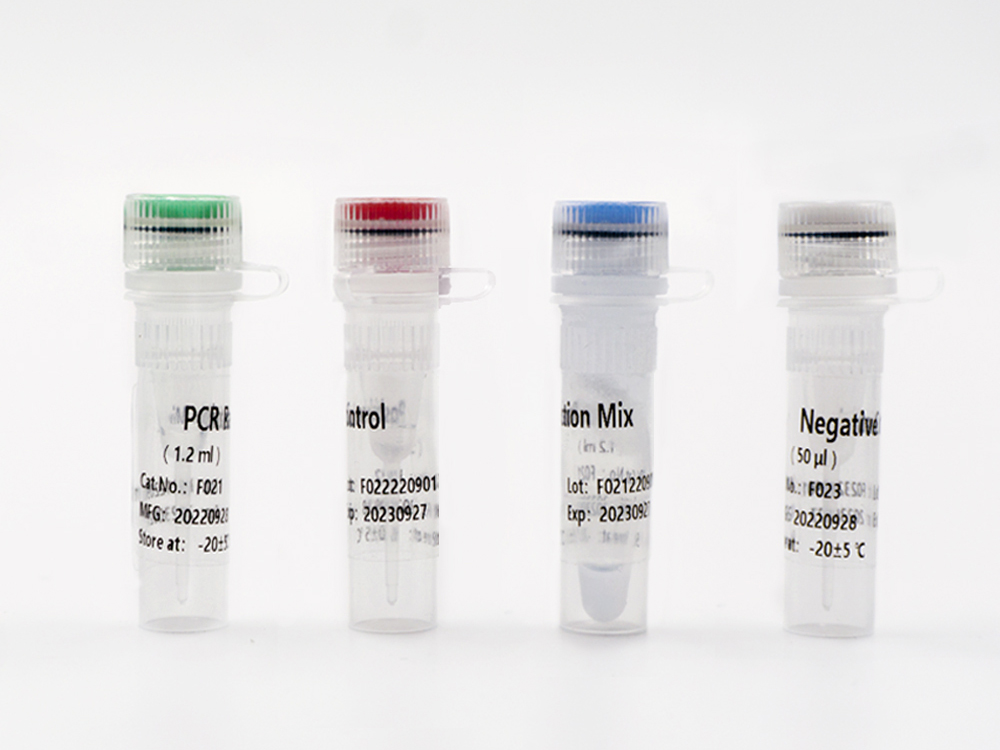
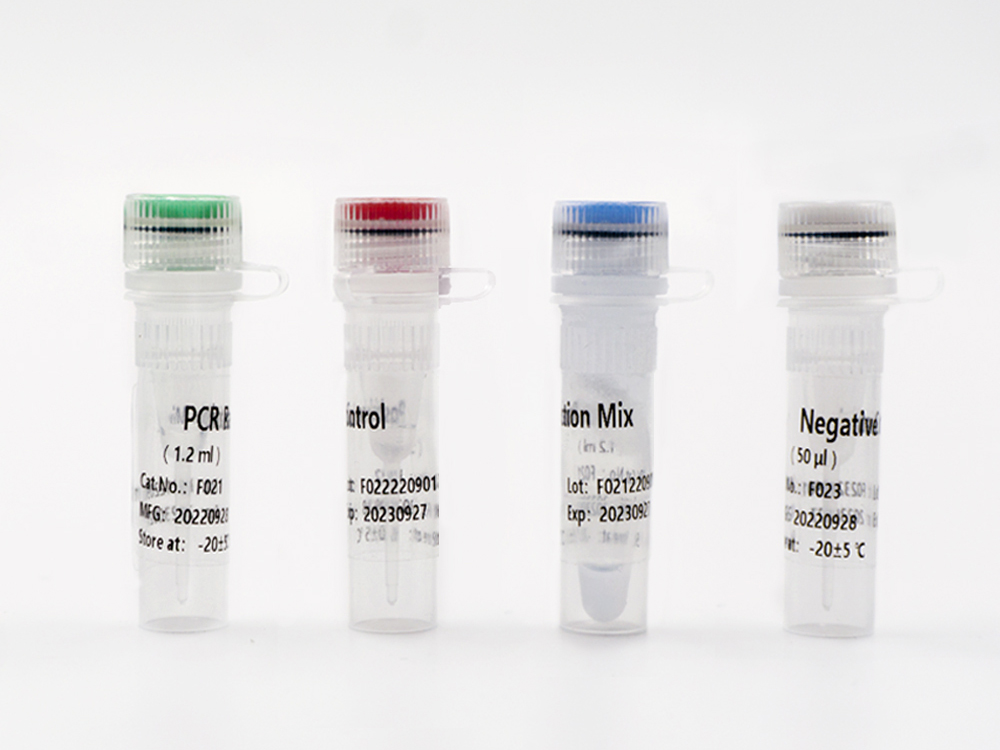
 This kit contains nucleic acid extraction reagent and PCR detection reagent. Nucleic acid is extracted by magnetic-bead-based method. This kit is based on the principle of fluorescence quantitative PCR method, using methylation-specific real-time PCR reaction to analyze template DNA, and simultaneously detect the CpG sites of PCDHGB7 gene and the quality control marker internal reference gene fragments G1 and G2. The methylation level of PCDHGB7 in the sample, or the Me value, is calculated according to the PCDHGB7 gene methylated DNA amplification Ct value and the Ct value of the reference. The PCDHGB7 gene hypermethylation positive or negative status is determined according to the Me value.
This kit contains nucleic acid extraction reagent and PCR detection reagent. Nucleic acid is extracted by magnetic-bead-based method. This kit is based on the principle of fluorescence quantitative PCR method, using methylation-specific real-time PCR reaction to analyze template DNA, and simultaneously detect the CpG sites of PCDHGB7 gene and the quality control marker internal reference gene fragments G1 and G2. The methylation level of PCDHGB7 in the sample, or the Me value, is calculated according to the PCDHGB7 gene methylated DNA amplification Ct value and the Ct value of the reference. The PCDHGB7 gene hypermethylation positive or negative status is determined according to the Me value.
Prognostic population
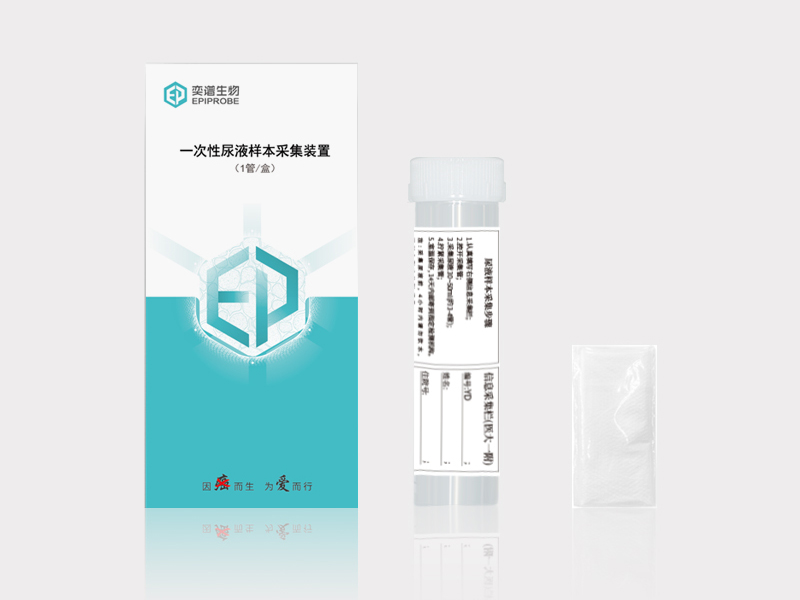
Sampling method: Place the disposable cervical sampler at the cervical os, gently rub the cervical brush and rotate 4-5times clockwise, slowly remove the cervical brush, put it into cell preservation solution, and label it for the following examination. Preservation of samples: Samples can be stored at room temperature for up to 14 days, at 2-8 ℃ for up to 2 months, and at -20±5℃ for up to 24 months.Early screening for healthy population: Endometrial cancer and precancerous lesions can be accurately screened out; Risk assessment for high-risk population: Risk assessment can be carried out for people with abnormal vaginal bleeding and endometrial thickening after menopause to assist in clinical diagnosis; Prognostic population recurrence monitoring: Postoperative population recurrence monitoring can be performed to prevent delays in treatment caused by recurrence.Healthy people
High-risk groups (people with abnormal vaginal bleeding after menopause, endometrial thickening, etc.)
 Epiprobe has a comprehensive infrastructure construction: GMP production center covers an area of 2200 square meters, and maintain an ISO13485 quality management system, which meets the production requirements of all types of genetic testing reagent products; the medical laboratory covers an area of 5400 square meters and has the ability to carry out cancer methylation detection business as a certified third-party medical laboratory. Besides, we have three products obtained CE certification, covering cervical cancer, endometrial cancer and urothelial cancer related detection. Epiprobe’s cancer molecular detection technology can be used for early cancer screening, auxiliary diagnosis, preoperative and postoperative evaluation, recrudescence monitoring, which runs through the whole process of cancer diagnosis and treatment, providing better solutions for doctors and patients.
Epiprobe has a comprehensive infrastructure construction: GMP production center covers an area of 2200 square meters, and maintain an ISO13485 quality management system, which meets the production requirements of all types of genetic testing reagent products; the medical laboratory covers an area of 5400 square meters and has the ability to carry out cancer methylation detection business as a certified third-party medical laboratory. Besides, we have three products obtained CE certification, covering cervical cancer, endometrial cancer and urothelial cancer related detection. Epiprobe’s cancer molecular detection technology can be used for early cancer screening, auxiliary diagnosis, preoperative and postoperative evaluation, recrudescence monitoring, which runs through the whole process of cancer diagnosis and treatment, providing better solutions for doctors and patients.